Background: Nicotine addiction remains a significant public health issue, with its seemingly mild reinforcing properties contrasting sharply with its high abuse rates. This project investigates the multifaceted nature of nicotine as a weak primary reinforcer but a powerful substance of abuse. The research delves into the complex interplay of genetic, biological, and environmental factors contributing to nicotine addiction, focusing on associative learning processes and the underlying neural mechanisms.
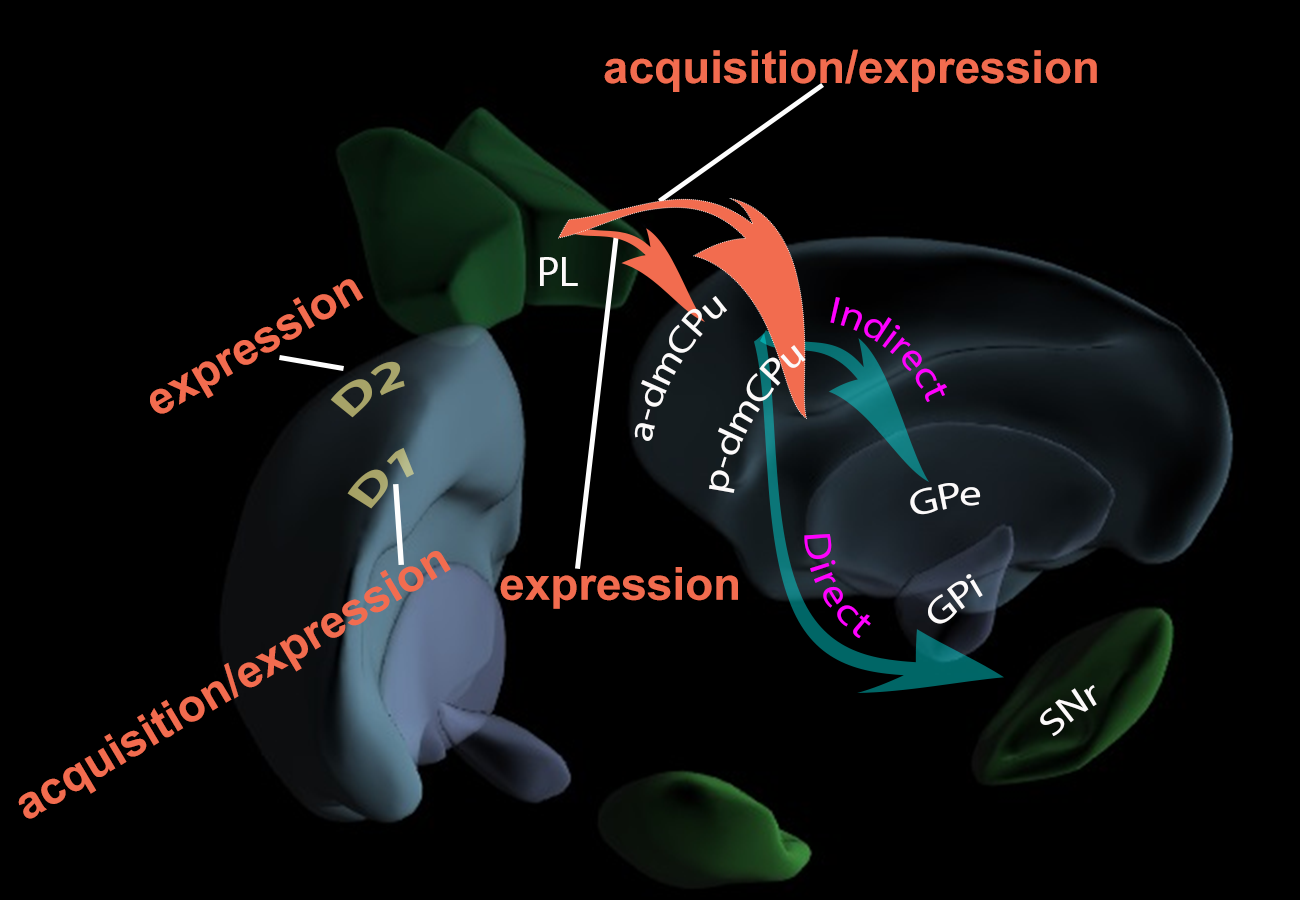
Research Objectives: The primary aim is to elucidate the role of the dorsomedial caudate-putamen (dmCPu) in learning associated with nicotine administration. This involves dissecting the afferent and efferent connections of the dmCPu, particularly focusing on the prelimbic cortex (PL) projections to dmCPu and their role in associative learning with nicotine as an interoceptive stimulus. The project also seeks to understand the distinct contributions of D1 and D2 receptors in the dmCPu to the associative learning processes related to nicotine use.
Hypothesized pathway and receptor model.
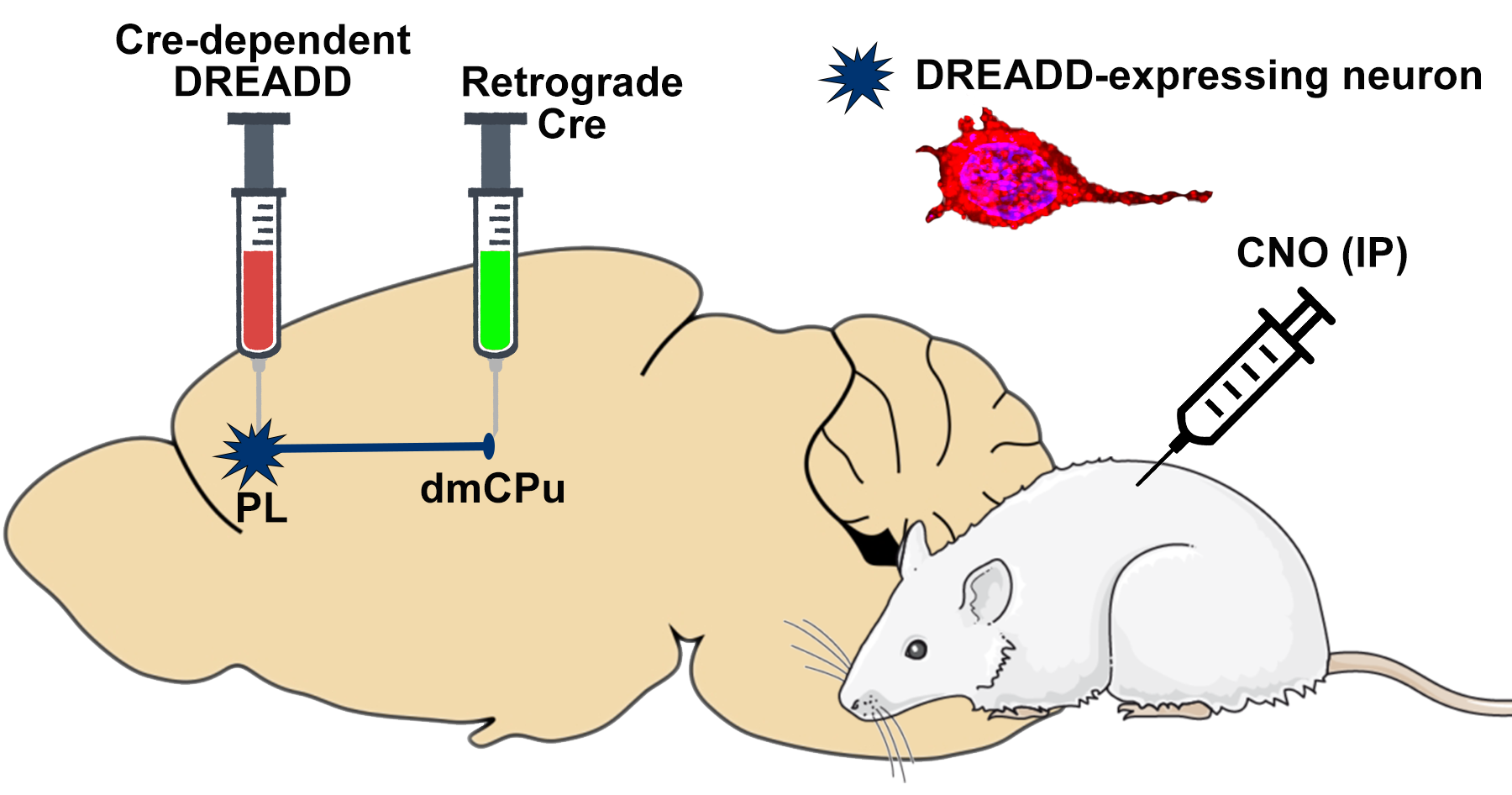
Methodology: Employing a rodent model, the study uses a modified nicotine self-administration paradigm to mimic human associative learning with nicotine. Rats are conditioned to associate nicotine intake with a subsequent reward, allowing for the examination of changes in behavior and neural activity. Advanced chemogenetic techniques are utilized to manipulate specific neural pathways involved in learning and addiction, providing insights into the causal relationships between neural activity and behavior.
Schematic representation of dual-vector approach to target corticostriatal projections.
Innovation and Impact: This research stands at the forefront of addiction studies by combining behavioral, pharmacological, and chemogenetic methodologies to address the underlying mechanisms of nicotine addiction. The findings aim to contribute to a better understanding of the neurobiological basis of addiction, potentially informing the development of more effective treatment strategies for nicotine dependence and other substance use disorders.
Long-term Vision: By unraveling the complex neurobiological underpinnings of nicotine addiction, the study aspires to pave the way for novel interventions targeting the associative learning mechanisms and neural circuits implicated in substance abuse. The long-term goal is to reduce the prevalence and impact of addiction in society, improving public health outcomes on a global scale.
References
2021
-
Inactivation of posterior but not anterior dorsomedial caudate-putamen impedes learning with self-administered nicotine stimulus in male rats
Christopher L. Robison, Theodore Kazan, Rikki L. A. Miller, and 2 more authors
Behavioural Brain Research, Sep 2021
The rodent caudate-putamen is a large heterogeneous neural structure with distinct anatomical connections that differ in their control of learning processes. Previous research suggests that the anterior and posterior dorsomedial caudate-putamen (a- and p-dmCPu) differentially regulate associative learning with a non-contingent nicotine stimulus. The current study used bilateral NMDA-induced excitotoxic lesions to the a-dmCPu and p-dmCPu to determine the functional involvement of a-dmCPu and p-dmCPu in appetitive learning with contingent nicotine stimulus. Rats with a-dmCPu, p-dmCPu, or sham lesions were trained to lever-press for intravenous nicotine (0.03 mg/kg/inf) followed by access to sucrose 30 s later. After 1, 3, 9, and 20 nicotine-sucrose training sessions, appetitive learning in the form of a goal-tracking response was assessed using a non-contingent nicotine-alone test. All rats acquired nicotine self-administration and learned to retrieve sucrose from a receptacle at equal rates. However, rats with lesions to p-dmCPu demonstrated blunted learning of the nicotine-sucrose association. Our primary findings show that rats with lesions to p-dmCPu had a blunted goal-tracking response to a non-contingent nicotine administration after 20 consecutive days of nicotine-sucrose pairing. Our findings extend previous reports to a contingent model of nicotine self-administration and show that p-dmCPu is involved in associative learning with nicotine stimulus using a paradigm where rats voluntarily self-administer nicotine infusions that are paired with access to sucrose—a paradigm that closely resembles learning processes observed in humans.
2017
-
Double dissociation of the anterior and posterior dorsomedial caudate-putamen in the acquisition and expression of associative learning with the nicotine stimulus
Sergios Charntikov, Steven T. Pittenger, Natashia Swalve, and 2 more authors
Neuropharmacology, Jul 2017
Tobacco use is the leading cause of preventable deaths worldwide. This habit is not only debilitating to individual users but also to those around them (second-hand smoking). Nicotine is the main addictive component of tobacco products and is a moderate stimulant and a mild reinforcer. Importantly, besides its unconditional effects, nicotine also has conditioned stimulus effects that may contribute to the tenacity of the smoking habit. Because the neurobiological substrates underlying these processes are virtually unexplored, the present study investigated the functional involvement of the dorsomedial caudate putamen (dmCPu) in learning processes with nicotine as an interoceptive stimulus. Rats were trained using the discriminated goal-tracking task where nicotine injections (0.4 mg/kg; SC), on some days, were paired with intermittent (36 per session) sucrose deliveries; sucrose was not available on interspersed saline days. Pre-training excitotoxic or post-training transient lesions of anterior or posterior dmCPu were used to elucidate the role of these areas in acquisition or expression of associative learning with nicotine stimulus. Pre-training lesion of p-dmCPu inhibited acquisition while post-training lesions of p-dmCPu attenuated the expression of associative learning with the nicotine stimulus. On the other hand, post-training lesions of a-dmCPu evoked nicotine-like responding following saline treatment indicating the role of this area in disinhibition of learned motor behaviors. These results, for the first time, show functionally distinct involvement of a- and p-dmCPu in various stages of associative learning using nicotine stimulus and provide an initial account of neural plasticity underlying these learning processes.
2012
-
Conditioned response evoked by nicotine conditioned stimulus preferentially induces c-Fos expression in medial regions of caudate-putamen
Sergios Charntikov, Matthew E. Tracy, Changjiu Zhao, and 2 more authors
Neuropsychopharmacology, Jul 2012
Publisher: Nature Publishing Group
Nicotine has both unconditioned and conditioned stimulus properties. Conditioned stimulus properties of nicotine may contribute to the tenacity of nicotine addiction. The purpose of this experiment was to use neurohistochemical analysis of rapidly developing c-Fos protein to elucidate neurobiological loci involved in the processing of nicotine as an interoceptive conditioned stimulus (CS). Rats were injected (SC) in an intermixed fashion with saline or nicotine (16 sessions of each) and placed in conditioning chambers where they were given one of the three conditions depending on group assignment: (a) nicotine paired 100% of the time with intermittent access to sucrose (nicotine-CS condition), (b) nicotine and saline each paired 50% of the time with sucrose (chamber-CS condition), or (c) no sucrose US control (CS-alone condition). Rats in the nicotine-CS condition acquired the discrimination as evidenced by goal-tracking (ie, increased dipper entries before initial sucrose delivery) only on nicotine sessions. The chamber-CS condition showed goal-tracking on all sessions; no goal-tracking was seen in the CS-alone condition. On the test day, rats in each condition were challenged with saline or nicotine and later assessed for c-Fos immunoreactivity. In concordance with previous reports, nicotine induced c-Fos expression in the majority of areas tested; however, learning-dependent expression was specific to dorsomedial and ventromedial regions of caudate-putamen (dmCPu, vmCPu). Only rats in the nicotine-CS condition, when challenged with nicotine, had higher c-Fos expression in the dmCPu and vmCPu. These results suggest that medial areas of CPu involved in excitatory conditioning with an appetitive nicotine CS. © 2012 American College of Neuropsychopharmacology. All rights reserved.